A Transition State of D3h symmetry was located computationally in a Density Functional Theory B3LYP calculation (pV5Z basis set, Gaussian 03) with an energy barrier of 21.6 kcal/mol. One imaginary frequency was obatined from the calculation at -98.54i cm-1. The vibrational mode was saved as an xyz file in Jmol. A 3D animation of the vibrational frequency that illustrates the Bailar Twist Mechanism is provided on the previous page.
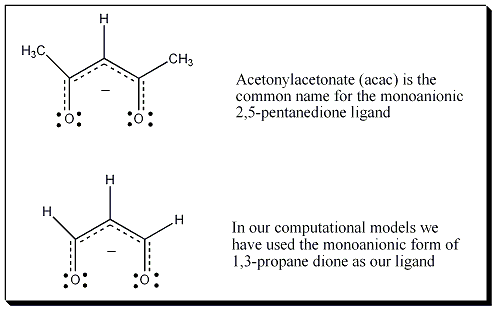
In this computation and animation 1,3-propanedione serves as an analog for the ubiquitous and well known acac (acetylacetonate = 2,5-pentanenedione) ligand.
The Bailar Twist Mechanism (and The Rây-Dutt mechanism for the interconversion of the &Delta and &Lambda isomers has been examined experimentally by NMR for similar GaL3 species.
In one study (where L = substituted catechols) the Bailar Twist was found to have a lower activation barrier (53 kJ/mol) than that of the Rây-Dutt (67 kJ/mol).
See Reference: Kersting, B., Telford, J.R., Meyer, M., Raymond, K.N.; J. Am. Chem. Soc., Vol 118, 1996, p. 5712-5721, DOI.
AND Davis, A.V., Firman, T.K., Hay, B.P, Raymond, K.N.; J. Am. Chem. Soc., Vol 128, 2006, p. 9484-9496, DOI
In a study with the GaL3 complex (L = fox = 5-fluoro-8-hydroxyquinoline), both the Bailar Twist and Rây-Dutt Mechanisms are observed with similar activation barriers of 17 kcal/mole (70.6 kJ/mol).
See Reference: Gromova, M., Jarjayes, O., Hamman. S., Nardin, R. Beguin, C. Willem, R.; European Journal of Inorganic Chemistry, 2000, 3, p. 545-550.