H. Exercises:
7. Contrasting Two Isomers of [Co(H2NCH2CH2NH2)3]3+
Isomer A:Do the ligands have &lambda or &delta configurations?
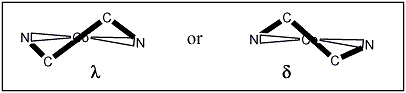
Decide if the other two ligands have &lambda or &delta configurations
If it helps:
Now test for C3 and C2 Operations:
Is the C3 Operation a Symmetry Operation for this molecule?
To test for a C2 you will need to align the molecule correctly with your mouse.
Does this isomer have one or more C2 Rotation Operations?
1. What is the point group for this molecule?
2. What is the configuration of each ligand?
3. Can you designate the metal complex as having a &Lambda or &Delta configuration?
4. What isomer would be the enantiomer of this isomer?
Do the ligands have &lambda or &delta configurations?

Decide if the other two ligands have &lambda or &delta configurations
If it helps:
Now test for C3 and C2 Operations:
Is the C3 Operation a Symmetry Operation for this molecule?
To test for a C2 you will need to align the molecule correctly with your mouse.
Does this isomer have one or more C2 Rotation Operations?
5. What is the point group for this molecule?
6. Is this molecule chiral?
7. What is the configuration of each ligand and the overall name of the isomer?
8. If this isomer is chiral, what would be the enantiomer of this isomer?