3. Stereochemical Properties of Metal Tris Chelates
C. Determining the Absolute Configuration (&Lambda or &Delta) for a Metal Tris Chelate
a. Method 1: ii) for Lambda (&Lambda)
We will illustrate two ways that we find most useful in determining the absolute configuration for a metal tris chelate. To illustrate we will use the &Lambda and &Delta Isomers of the Tris(acetonylacetonate)metal complexes
To your left is one of the isomers of Tris(acetonylacetonate)ruthenium(III) Reference
First: allign your molecule so that you are looking directly down the C3 axis:
Potentially useful commands:
Make sure one coordinating atom from each ligand is in the front and the second coordinating atom (from the same ligand) is in the back:
Now run the test with your left hand:
Place your finger tips on a back ligand atom and curl your fingers along the backbone of that same ligand toward the front coordinating atom (ie go from the green atom to the red atom along the ligand backbone).
If you can do this with your left hand and your thumb is pointing towards you; then you have the &Lambda Isomer
So here is the test with Marion's left hand:
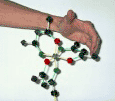
It works! Marion's thumb is pointing towards you, thus this is the &Lambda Isomer